Adult neurogenesis
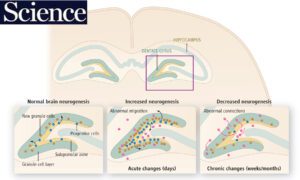
Adult neurogenesis is the birth of new neurons in the adult brain. Although most neurons are born prenatally, some are born after birth and in adulthood. Although those born in adulthood are few, they appear to be essential for normal brain function.
Adult neurogenesis is prominent in the dentate gyrus. The adult-born neurons mainly become excitatory granule cells, the primary cell type. We have been interested in the dentate gyrus because we found adult-born neurons play important roles there in the context of epilepsy.
In our first studies, we found adult-born neurons in the hilus, an abnormal location for granule cells ('ectopic' granule cells; Scharfman et al., J Neurosci, 2000). There the ectopic granule cells seemed to promote seizure activity, and after collaborating with the laboratory of Jeannie Hsieh, evidence was acquired to support that idea (Cho et al., Nat Med., 2015).
Later studies showed that normal adult-born granule cells — not the ectopic granule cells — had inhibitory effects on the granule cell population, at least when they were young (Drew et al., Hippocampus, 2015). This inhibitory effect was due to activation of GABAergic neurons. This work was part of a collaboration with the laboratory of Rene Hen.
Consistent with these inhibitory effects of young adult-born granule cells, we found that they inhibited seizures in the normal brain, and reduced damage caused by severe seizures (Iyengar et al., Exp. Neurol., 2015; Jain et al., Hippocampus, 2019).
Recent work suggests that deleting the normal adult-born cells can actually prevent epilepsy (Jain et al., eLife, in press).
Thus, there are diverse roles of adult-born granule cells. In the normal brain, those that are normal seem to be 'good' whereas adult-born ectopic cells in the epileptic brain seem 'bad.'
The CA3 "backprojection"
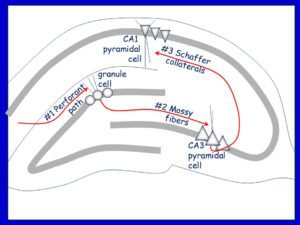
Top: The hippocampus has been considered to mainly be composed of excitatory neurons which receive their main input from the entorhinal cortical axons, the perforant path, which innervates the granule cells of the dentate gyrus. A so called trisynaptic circuit begins with this synapse (#1) and then is followed by excitation of CA3 (#2) and then CA1 (#3).
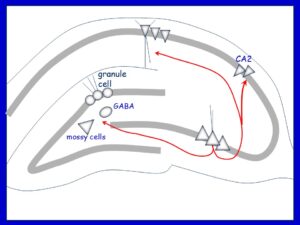
Bottom: An alternative view is "CA3-centric" where CA3 activates the dentate gyrus via "backprojections" to hilar mossy cells and GABAergic neurons which project to granule cells. CA3 also projects to CA1, CA2, and the contralateral hippocampus.
Our studies investigated anatomical reports that CA3 axons reached the hilus. We performed electrophysiology to show that CA3 activation led to excitation of hilar neurons and inhibition of granule cells. However, without the GABAergic neurons hilar mossy cells were able to activate granule cells. We also showed that CA3 neurons made direct projection to hilar mossy cells using simultaneous intracellular recordings of pyramidal cells and mossy cells.
Selected publications:
- Scharfman (1994) J Neurosci https://pubmed.ncbi.nlm.nih.gov/7931561/
- Scharfman (2007) Prog Br. Research https://pubmed.ncbi.nlm.nih.gov/17765742/
- Scharfman (1994) J Neurophysiol https://pubmed.ncbi.nlm.nih.gov/7884451/
- Scharfman (1996) Neuroscience https://pubmed.ncbi.nlm.nih.gov/9157312/
BDNF, estrogen and testosterone
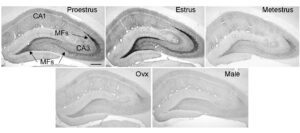
The Figure shows the results of staining in the hippocampus of the rat using an antibody to BDNF. Most staining is in the mossy fiber (MF) axons (arrows) of the dentate gyrus granule cells. The top row shows sections from female rats that either were studied when estrogen was high (proestrous morning), 24 hrs later (estrous morning) or 48 hrs later (metestrous morning). BDNF in the MFs increases on proestrous morning in the granule cells and then reaches a peak on estrous morning once BDNF has been anterogradely transported. The bottom row shows low levels of BDNF after ovariectomy (Ovx) and in a male.
In the hippocampus, brain-derived neurotrophic factor (BDNF) is considered critical to the synaptic plasticity of neurons, which enables the structure to play its critical role in learning and memory. The highest concentration of BDNF is in the granule cells of the dentate gyrus, where it is transported to the axon (mossy fiber, MF) terminals. After it was shown that estrogen could increase BDNF synthesis we found that MF BDNF increased in female rats when they entered the stage of the ovarian cycle when estrogen rose (proestrous morning). MF BDNF was even higher 24 hrs later (estrous morning) before returning to a relatively low level until the next rise in estrogen. The fluctuations were important because they led to increase excitability of the mossy fiber targets, the CA3 pyramidal cells. We als showed that ovariectomy (Ovx) reduced MF BDNF and estrogen treatment restored it. The estrogen-treated animals learned better than the vehicle-treated controls. We also showed that normal male rats did not show fluctuations in BDNF but after removing the testes BDNF rose and increased CA3 excitability.
Selected publications:
- Scharfman et al. (2003) J Neurosci. https://pubmed.ncbi.nlm.nih.gov/14684866/
- Scharfman et al. (2007) Eur. J. Neurosci. https://pubmed.ncbi.nlm.nih.gov/17970745/
- Skucas et al. (2013) J. Neurosci. https://pubmed.ncbi.nlm.nih.gov/23392664/
- Harte-Hargrove et al. (2015) J Neurosci. https://pubmed.ncbi.nlm.nih.gov/25632146/